CEMF Group Publishes Atomic Protein Structure
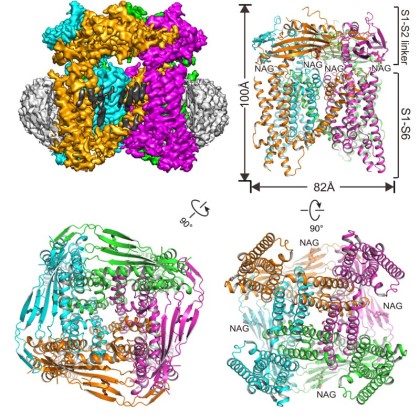
First publication of an atomic protein structure determined using the CEMF The collaborative effort of Dr. Youxing Jiang’s group (Physiology and Biophysics) and Dr. Xiao-chen Bai’s group (Biophysics and Cell biology) resulted in the world’s first 3D atomic structure of a mammalian ion channel Transient receptor potential mucolipin 1 (TRPML1) (Chen*, She* et al., Nature, 2017).
TRPML1 is an endo/lysosomal cation channel, and its loss-of-function mutations are the direct cause of Type IV mucolipidosis (MLIV), an autosomal recessive lysosomal storage disease. By using single particle cryo-electron microscopy (cryo-EM) in combination with mutagenesis, the TRPML1 structure reveals the PIP2 binding site and several key features in the selectivity filter that determine the cation ion selectivity. Through a particles sorting approach, the structure also reveals two equally distributed S4-S5 linker conformations in the closed channel, providing structural implication for the S4-S5 linker-mediated PIP2 gating mechanism among TRPML channels. This work marks the first publication of a structure determined using the university’s new $17 million Cryo-Electron Microscopy Facility (CEMF).
