Transfer RNA regulates messenger RNA degradation
Fundamental mechanism could eventually be harnessed to treat obesity, cancer, and other diseases, UTSW researchers say

DALLAS – Nov. 21, 2024 – Transfer RNA (tRNA), a genetic molecule well known for its contribution to reading the instructions for building proteins, also plays a key role in regulating how long those instructions persist in cells, a new study by UT Southwestern Medical Center researchers shows. The findings, published in Science, expand the understanding of the timing involved in the degradation of messenger RNA (mRNA), a vital mechanism for controlling gene activity, and could eventually have the potential to lead to new treatments for obesity, cancer, and other health conditions.
“Our work revealed a fundamental role for tRNAs in controlling mRNA stability and connecting the sequence of an mRNA to its rate of decay,” said Joshua T. Mendell, M.D., Ph.D., Professor of Molecular Biology at UT Southwestern and a Howard Hughes Medical Institute Investigator. Dr. Mendell co-led the study with Jan P. Erzberger, Ph.D., Associate Professor of Biophysics, and Xiaoqiang Zhu, Ph.D., Assistant Instructor of Molecular Biology.
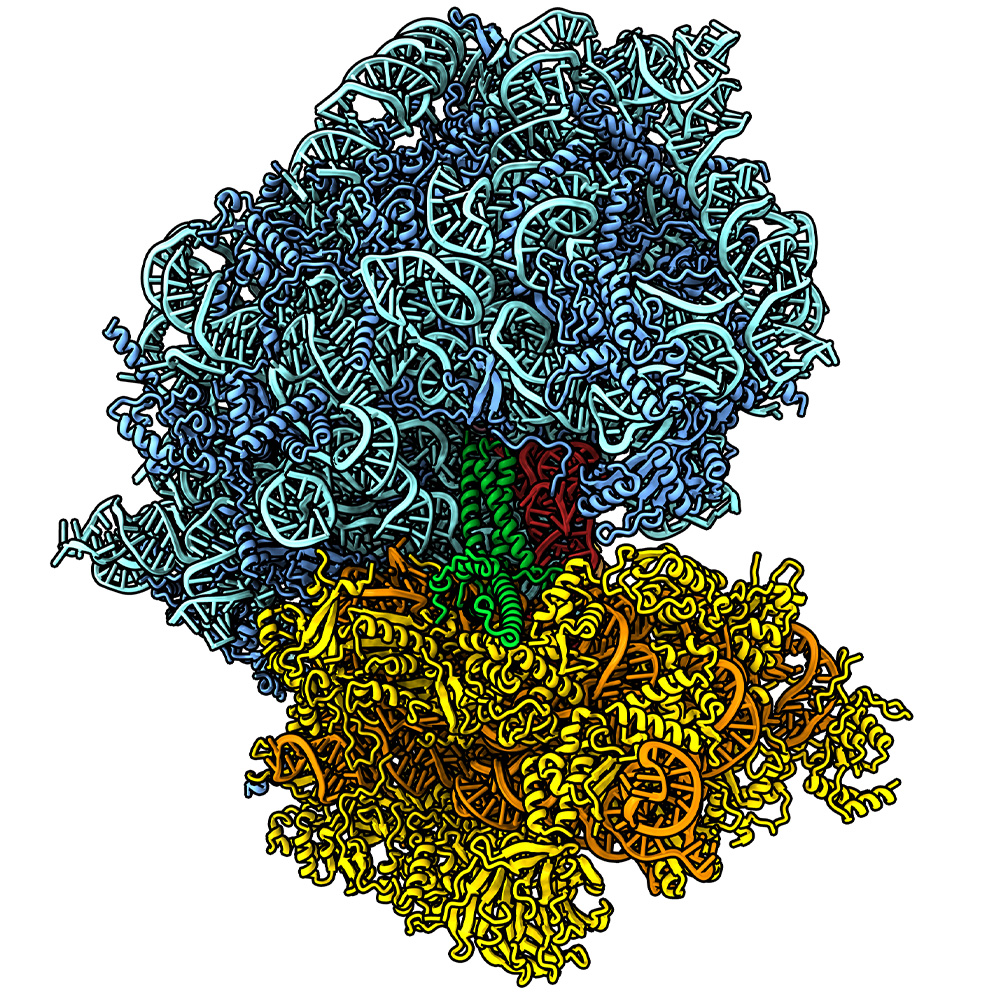
How long each mRNA transcript persists in cells is pivotal for determining how much of each protein is made, which in turn affects how cells function, Dr. Mendell explained. For example, mRNA vaccines – like those taken by billions of people worldwide to protect against the virus that causes COVID-19 – work best when their mRNA persists for a long time, causing cells to continue to churn out proteins that ready the immune system to attack. Conversely, mRNAs that produce proteins that edit DNA, such as CRISPR proteins that can correct genetic errors, need to be removed as soon as they finish their job so healthy DNA is not harmed by mistake.
Although it’s been known that a molecular complex called CCR4-NOT plays a central part in degrading mRNAs, how CCR4-NOT is recruited to specific mRNAs in human cells was unclear.
To answer this question, the Mendell Lab and the Erzberger Lab – which study different aspects of RNA biology using complementary methods – merged efforts. Together, the teams began identifying the mRNAs in human cells that were most strongly associated with the CCR4-NOT complex.
“We found that these mRNAs were highly enriched for the instructions, or codons, that tell the cell to add the amino acid arginine to the encoded protein,” Dr. Erzberger said.
Victor Emmanuel Cruz, Ph.D., Senior Research and Development Scientist who previously worked in the Erzberger Lab, performed structural studies in UTSW’s Cryo-Electron Microscopy Facility, confirming that when the cellular machines that synthesize proteins, called ribosomes, encountered one of three special arginine codons, the tRNAs that recognize these codons recruited the CCR4-NOT complex to begin the mRNA degradation process. When the researchers mutated the mRNA so it no longer encoded arginine, or mutated the tRNA so it could not recruit CCR4-NOT, the mRNA that would normally be degraded instead persisted in cells and more protein was produced.
The investigators found that mRNAs that produce proteins that are part of mitochondria – the power-producing organelles in cells – were most likely to have the decay-inducing arginine codons. Thus, impairing the recruitment of CCR4-NOT by tRNAs that recognize arginine codons caused cells to increase the abundance and activity of mitochondria.
Because mitochondrial mRNAs were most heavily affected by this newly identified degradation signal, Dr. Mendell said, researchers may eventually be able to harness this mechanism to treat certain inherited mitochondrial disorders as well as other diseases in which mitochondria play a critical role, such as obesity and cancer.
He Zhang, Ph.D., Computational Biologist at UTSW, also contributed to this study.
Dr. Mendell holds the Charles Cameron Sprague, M.D. Chair in Medical Science and is a member of the Harold C. Simmons Comprehensive Cancer Center.
This research was funded by grants from the National Institutes of Health (R01CA282036 and GM135617-01), the Cancer Prevention and Research Institute of Texas (CPRIT) (RP220309, RR150074, and RP170644), The Welch Foundation (I-1961 and I-1897), and the UTSW Endowed Scholars Fund. The UT Southwestern Cryo-Electron Microscopy Facility is supported by grant RP220582 from CPRIT.
About UT Southwestern Medical Center
UT Southwestern, one of the nation’s premier academic medical centers, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty members have received six Nobel Prizes and include 25 members of the National Academy of Sciences, 24 members of the National Academy of Medicine, and 14 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 3,200 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in more than 80 specialties to more than 120,000 hospitalized patients, more than 360,000 emergency room cases, and oversee nearly 5 million outpatient visits a year.
