New structure that mimics blastocysts could aid research into early human development
DALLAS – March 22, 2021 – A UT Southwestern research team has generated biological structures that resemble blastocysts, the structures that form from the early development of fertilized eggs in mammals, using previously established human embryonic stem cells derived from embryos donated for research and human-induced pluripotent stem cells generated from adult cells – collectively known as human pluripotent stem cells.
The findings, published online today in Nature, could offer a new way to study early human development, pregnancy loss, and developmental defects. Despite their similar morphology to blastocysts, these structures cannot develop into fetuses, says study leader Jun Wu, Ph.D., an assistant professor of molecular biology at UTSW.
“Having an in vitro biological model for blastocyst development is critically important to fill the gap for understanding human development without relying extensively on human embryos,” Wu says.
Human pluripotent stem cells – cells at one of the earliest stages of development – have the potential to become nearly all of the body’s many tissue types. However, it was not known what molecular signals were important to get them to develop into blastocysts, hollow ball-shaped early embryos that form about five days after conception before implanting into the uterine wall. Studies of this stage of human development have largely relied on discarded/donated embryos from fertility treatments, a scarce resource that has ethical concerns, Wu says.
Blastocysts contain three main cell types: epiblasts, hypoblasts, and trophoblasts. Epiblasts are the quintessential embryonic stem cell, explains Wu, forming various mature tissue types. Consequently, researchers have long maintained cell lines of epiblasts for research. Recent studies have shown that by exposing epiblasts to chemicals that activate the right combinations of genes, these cells can develop into hypoblast or trophoblast cells.
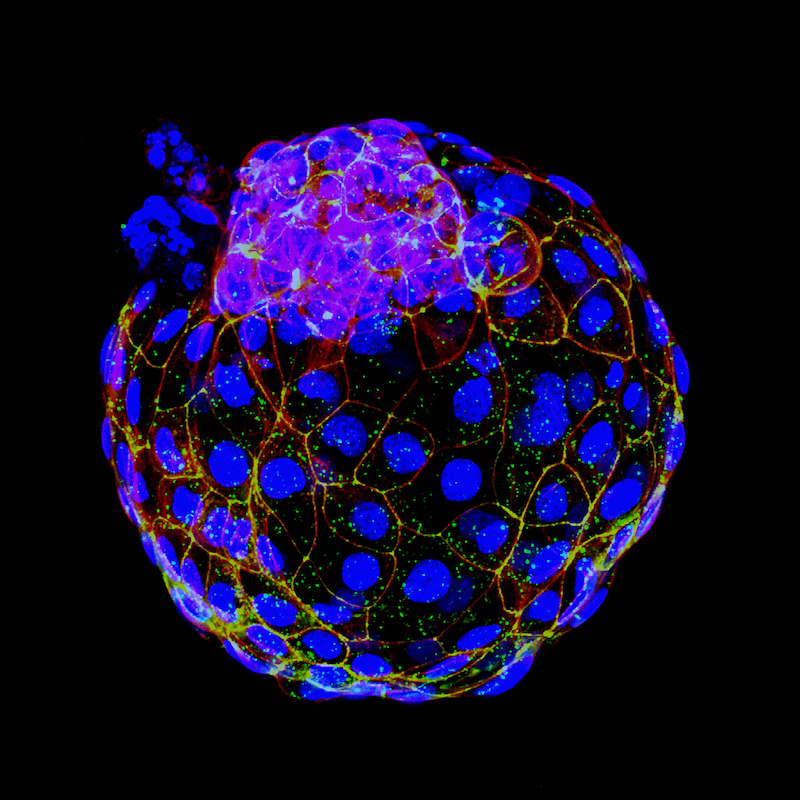
Building on these findings, Wu and his colleagues, including Gary Hon, Ph.D., assistant professor of obstetrics and gynecology and in the Lyda Hill Department of Bioinformatics at UTSW, used similar techniques to encourage epiblast cells to form entire blastocyst-like structures with all three blastocyst cell types present. The researchers treated human embryonic stem cells in cell culture dishes first with chemicals to activate the molecular pathways TGF-beta, FGF, and WNT that have been shown to steer development into hypoblasts, then with chemicals that shut these same pathways down, which guided development toward trophoblasts. In some experiments, they also switched the order in which these chemicals were administered. In both cases, the embryonic stem cells formed cavity-containing structures that looked much like human blastocysts. When Wu and his colleagues tried a similar method with induced pluripotent stem cells – adult cells that have been reprogrammed to regain embryonic stem cell-like qualities – they had similar results.
Analysis showed that these structures, which the researchers call human blastoids, matched the physical characteristics of human blastocysts – they had the same shape, size, and number of cells. A survey of lineage marker proteins showed that the trophoblasts, hypoblasts, and epiblasts were in the same places as found in natural blastocysts. Tests of global gene expression, performed by Hon’s laboratory, showed that genes activated or silenced in the blastoids roughly matched those in human embryos at the same stage of development.
Much like human blastocysts, the researchers could derive stem cells from each cell type in the blastoids. When Wu and his colleagues followed the blastoids into a slightly later stage of development, about 10 days old, they began forming amniotic cavity and yolk sacs and secreting human chorionic gonadotropin, characteristics that human embryos also display.
As proof of principle that these blastoids can be used for research related to human development, the researchers treated these structures with chemicals that inhibit different protein kinase C (PKC) isozymes, proteins thought to be important for the blastocyst cavity to develop. Sure enough, blastoids that received inhibitors for δ, ζ and η isozymes of PKC didn’t form their characteristic cavities.
Wu says this finding suggests that these structures could make good models for better understanding environmental or other chemicals that disrupt early human development. They could also be used to better understand gene activity in embryos or what factors affect successful implantation. Although the efficiency of generating structures of later stage of development from blastoids is low, he adds, further research can help refine this process to create these structures more robustly.
“Right now, much about this stage of human development is a black box,” Wu says. “Blastoids could eventually provide an unlimited resource for better understanding the workings of human blastocysts.”
Hon is a member of UTSW’s Cecil H. and Ida Green Center for Reproductive Biology Sciences.
Other researchers who contributed to this study include Leqian Yu, Yulei Wei, Jialei Duan, Daniel A. Schmitz, Masahiro Sakurai, and Lei Wang, all of UTSW; and Kunhua Wang and Shuhua Zhao of The First Affiliated Hospital of Kunming Medical University.
Wu is a Virginia Murchison Linthicum Scholar in Medical Research who is funded by the Cancer Research and Prevention Institute of Texas (CPRIT) (RR170076) and the Hamon Center for Regenerative Science and Medicine at UT Southwestern. Hon is supported by CPRIT (RP190451), the National Institutes of Health (DP2GM128203), The Welch Foundation (I-1926-20170325), the Burroughs Wellcome Fund (1019804), and the Green Center for Reproductive Biology Sciences.
