UT Southwestern study shows glucagon is key for kidney health
Deleting receptors for hormone known for boosting blood sugar produces symptoms that mimic chronic kidney disease
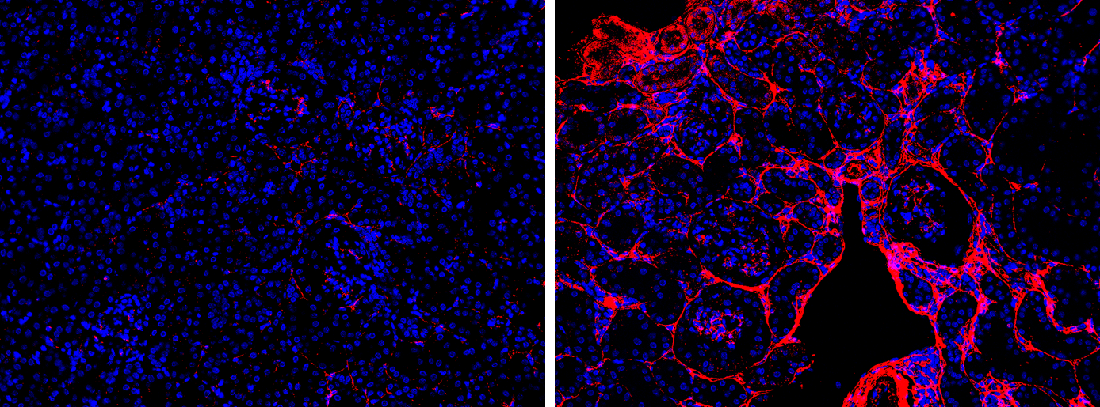
DALLAS – Feb. 22, 2024 – Glucagon, a hormone best known for promoting blood sugar production in the liver, also appears to play a key role in maintaining kidney health. When UT Southwestern Medical Center researchers removed receptors for this hormone from mouse kidneys, the animals developed symptoms akin to chronic kidney disease (CKD).

Their findings, published in Cell Metabolism, shed new light on glucagon’s physiological functions and provide new insights into CKD, a disease that affects hundreds of millions of people around the globe, according to the National Institute of Diabetes and Digestive and Kidney Diseases.
“Our study defines important protective effects of glucagon for kidney health and normal systemic metabolic well-being of the entire organism,” said study leader Philipp Scherer, Ph.D., Professor of Internal Medicine and Cell Biology and Director of UTSW’s Touchstone Center for Diabetes Research.
Over the past century, researchers have discovered that cells in the pancreas produce glucagon when blood sugar, or glucose, dips below a certain threshold. This hormone migrates through the bloodstream to receptors on the surface of liver cells, prompting the liver to produce glucose that fuels cells throughout the body. More recent research has shown that the kidneys also bear glucagon receptors, but besides stimulating production of a minor amount of glucose, their role has been unclear, Dr. Scherer explained.
To better understand the function of these kidney-based glucagon receptors, Dr. Scherer and his colleagues used genetic techniques to eliminate the receptors in mice and compared them to mice without genetic manipulations and others with glucagon receptors deleted in the liver.
Unlike the other two groups, the mice that had glucagon receptors removed in the kidneys showed a host of pathologies that plagued this organ. These included inflammation, scarring, and excess lipid deposits similar to what is seen in fatty liver disease, as well as high blood pressure and associated kidney-related damage, alterations in the activity of energy-production genes, and signs of high oxidative stress.

Mice without kidney-based glucagon receptors also had a range of deficits stemming from kidney dysfunction that affected their entire bodies, such as a dysregulation of nitrogen, problems maintaining water and electrolyte balances, and heart problems.
These issues largely mimic those in patients with CKD, noted May-Yun Wang, Ph.D., Assistant Professor of Internal Medicine and first author of the study. Studies have shown that individuals with CKD have fewer kidney glucagon receptors, although it’s unclear which occurred first – kidney pathology that decreased receptor numbers or pathology that arose from an insufficient amount of receptors. This question is a topic for future research, Dr. Wang said.
In the meantime, she added, newer drugs in late-stage clinical trials to treat obesity and diabetes are incorporating glucagon, a strategy found to aid in weight loss that could also unexpectedly benefit patients with CKD.
“These drugs are already showing improvements to kidney health in clinical trials, and our findings provide an explanation why,” Dr. Scherer said.
Other UTSW researchers who contributed to this study are Laurent Gautron, Ph.D., Assistant Professor of Internal Medicine; Denise K. Marciano, M.D., Ph.D., Associate Professor of Internal Medicine and Cell Biology; Ruth Gordillo, Ph.D., Associate Professor of Internal Medicine; Qingzhang Zhu, Ph.D., and Chao Li, Ph.D., both Instructors of Internal Medicine; Xue-Nan Sun, Ph.D., postdoctoral fellow; Shiuhwei Chen, M.S., Senior Research Associate; and Megan Paredes, M.S., Research Associate.
This study was funded by National Institutes of Health grants (RC2-DK118620, R01-DK55758, R01-DK099110, R01-DK127274, R01-DK131537, and R00-AG068239), a Voelcker Fund Young Investigator Pilot Grant, an American Heart Association Career Development Award (855170), and a Canadian Institutes of Health Research grant (154321).
Dr. Scherer holds the Gifford O. Touchstone, Jr. and Randolph G. Touchstone Distinguished Chair in Diabetes Research and the Touchstone/West Distinguished Chair in Diabetes Research. Dr. Marciano holds the Carolyn R. Bacon Distinguished Professorship in Medical Science and Education.
About UT Southwestern Medical Center
UT Southwestern, one of the nation’s premier academic medical centers, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty members have received six Nobel Prizes and include 25 members of the National Academy of Sciences, 21 members of the National Academy of Medicine, and 13 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 3,100 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in more than 80 specialties to more than 120,000 hospitalized patients, more than 360,000 emergency room cases, and oversee nearly 5 million outpatient visits a year.
