Experimental compound extends life in ALS mouse model
Molecule discovered by UTSW researchers could lead to new therapies for devastating motor neuron disease

DALLAS – Feb. 06, 2024 – UT Southwestern Medical Center researchers have identified an experimental molecular compound that improved survival among cellular models and mouse models of amyotrophic lateral sclerosis (ALS), the fatal neurodegenerative disease. Their findings, reported in Cell Death & Disease, offer promise for the potential development of treatments for ALS, which has no effective therapy.
“This study will significantly advance the ALS field by providing a leading compound and a signaling pathway for future investigations,” said study leader Chun-Li Zhang, Ph.D., Professor of Molecular Biology and a W.W. Caruth, Jr. Scholar in Biomedical Research at UT Southwestern. Dr. Zhang is also an Investigator in the Peter O’Donnell Jr. Brain Institute at UTSW.
ALS, also known as Lou Gehrig’s disease, affects hundreds of thousands worldwide. With onset in midlife, ALS kills motor neurons over time, gradually depriving patients of the ability to walk, talk, swallow, and breathe. Life expectancy is two to five years after diagnosis and hasn’t changed despite decades of research, Dr. Zhang explained.
Searching for potential therapies that might extend the lives of ALS patients, Dr. Zhang and his colleagues tested compounds from a pharmaceutical library on a cellular model of ALS. Because it is impossible to sample motor neurons directly from ALS patients, previous studies have largely used neurons that were derived from pluripotent stem cells. However, Dr. Zhang said, these neurons were reset to an embryonic stage, losing age-related changes.
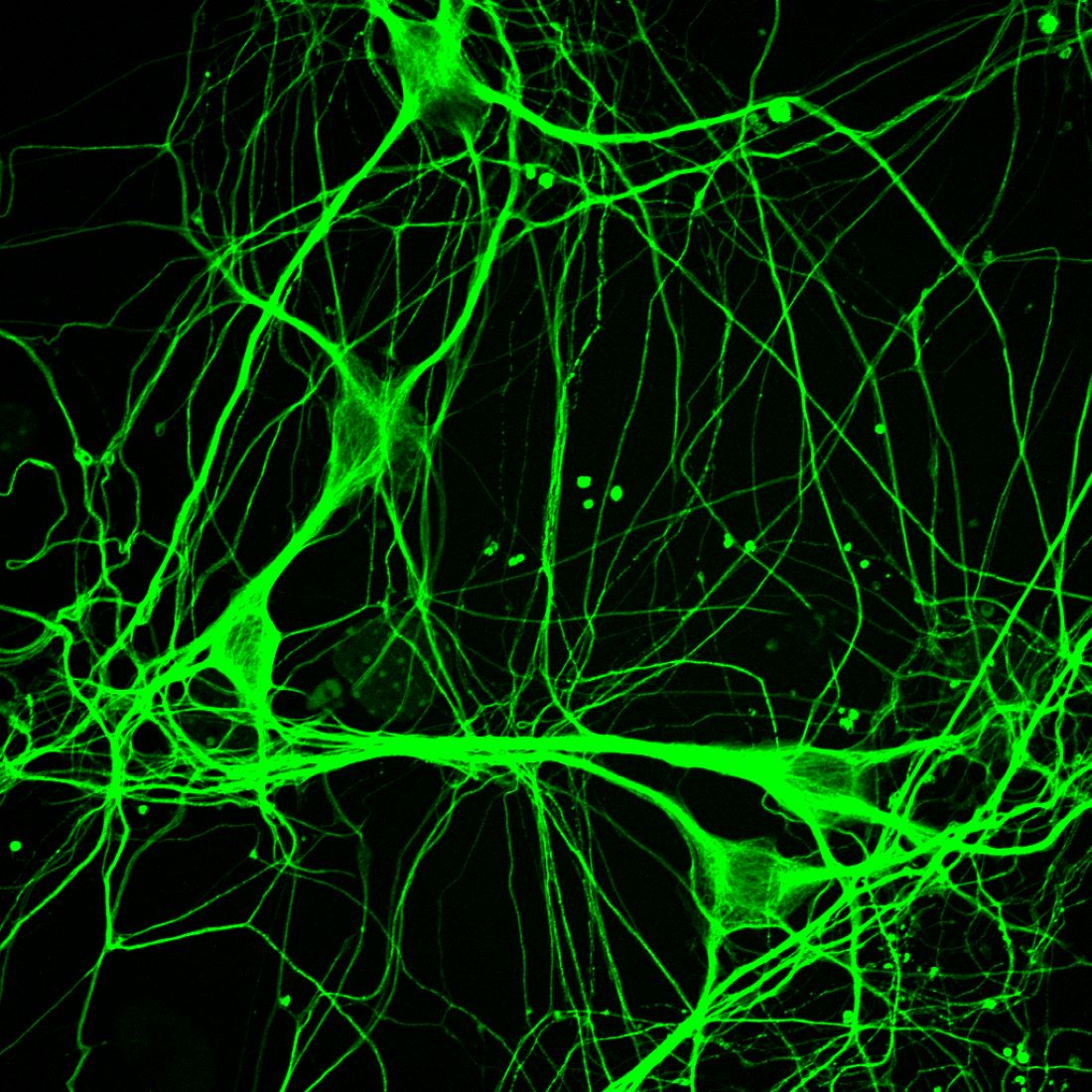
For the new study, the researchers used a different approach that converted ALS patients’ skin cells into motor neurons that bore marks of aging, providing a more realistic model. After dosing these cells with about 2,000 compounds, the researchers identified a promising one they dubbed Hit3. This compound reversed some of the cells’ ALS-related morphological changes, causing them to grow larger cell bodies, develop more complicated branching in their extensions, form more connections with muscle cells, and live significantly longer than cells that didn’t receive Hit3.
A closer look showed that Hit3 acts on cell proteins called MAP4Ks, which play key roles in cells’ responses to stress. Once activated, MAP4Ks regulate a cascade of other proteins involved in this role, a molecular pathway that appears critical in deciding whether motor neurons live or die and has been implicated in ALS and other neurodegenerative diseases.
To determine what effect manipulating this pathway could have on ALS animal models, the researchers dosed mice that had mutations in a gene called SOD1 – considered the most aggressive form of ALS – with a compound related to Hit3 called MAP4Ki. Mice that didn’t receive this compound had a dramatic loss of motor neurons and died at an average of 129 days. However, mice that received MAP4Ki maintained significantly more motor neurons and lived 10 days longer.
“Even though MAP4Ki extended survival by just a short time, our results suggest treatments that block the MAP4K pathway could one day be useful therapeutically,” Dr. Zhang said.
Because MAP4Ki isn’t optimized for pharmaceutical use – it degrades quickly and can’t cross the blood-brain barrier, limiting its absorption – this compound has significant room for chemical manipulations that could improve its activity, Dr. Zhang said. In addition, he noted, because targeting the MAP4K pathway has shown promise, researchers could eventually develop other drugs designed to affect this pathway more successfully. The hope is that this could potentially extend ALS patients’ life span.
Other UTSW researchers who contributed to this study were Shuaipeng Ma, Ph.D., Research Scientist; Wenjiao Tai, M.D., Ph.D., Instructor of Molecular Biology; Xiaoling Zhong, Ph.D., Research Scientist; and Yuhua Zou, M.Sc., Research Scientist, all members of the Zhang Lab.
About UT Southwestern Medical Center
UT Southwestern, one of the nation’s premier academic medical centers, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty members have received six Nobel Prizes and include 26 members of the National Academy of Sciences, 21 members of the National Academy of Medicine, and 13 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 3,100 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in more than 80 specialties to more than 120,000 hospitalized patients, more than 360,000 emergency room cases, and oversee nearly 5 million outpatient visits a year.
