UTSW research team “retrieves” structure of protein recycling complex implicated in cancer
Cells engulf parts of their membranes for many purposes: to transport large molecules, to “eat” bacteria or debris from dead cells, or to “drink” external fluid, for example. This process, called endocytosis, also captures important proteins in the cell’s surface. To maintain normal function, cells must cycle these proteins back to the surface by a complex and not fully understood process.
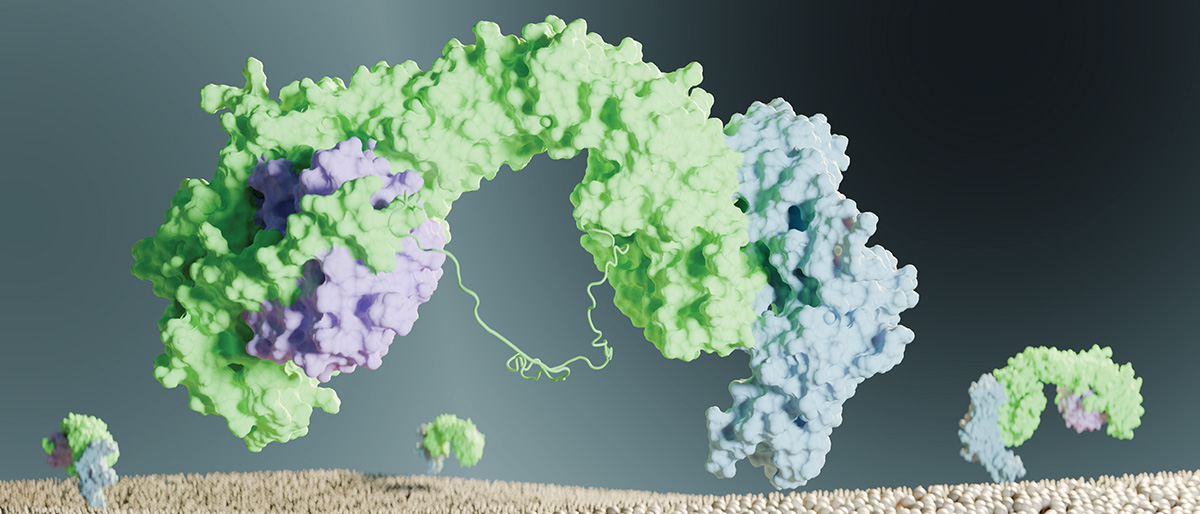
Taking a major step to understand this system, UT Southwestern researchers uncovered the structure of a protein recycling complex called retriever-CCC. They found that this structure is conserved among species, meaning that it is evolutionarily ancient. They also showed that cancer-associated mutations disrupt it.
“Understanding protein structures helps us see how mutations can affect the functions of a protein,” said Ezra Burstein, M.D., Ph.D., Professor of Internal Medicine and Molecular Biology and co-senior author of the study, which was published in Nature Structural & Molecular Biology. “This is a watershed moment in understanding this system.”
This work brought together the knowledge of cell biology in the Burstein Lab with the expertise in structural biology of Baoyu “Stone” Chen, Ph.D., Associate Professor of Biochemistry at Iowa State University, and Zhe “James” Chen, Ph.D., Professor of Biophysics and co-Director of the Structural Biology Laboratory at UT Southwestern, all co-senior authors in this work.

“Knowing the structure can also allow us to think how to use pharmacology to activate or inactivate a protein for treatment purposes,” said Dr. Burstein, also Chief of the Division of Digestive and Liver Diseases and a member of the Harold C. Simmons Comprehensive Cancer Center.
For instance, structural analysis can reveal which parts of a protein are folded within it – inaccessible to potential drugs – and which portions are exposed and able to interact with their surroundings.
Dr. Burstein and his colleagues co-discovered retriever in 2017; they found that retriever – composed of three proteins called VPS26C, VPS35L, and VPS29 – binds to more than 120 surface proteins to help maintain them at the correct concentration on the cell surface. These surface proteins include the low-density cholesterol receptor, which binds to and removes “bad” cholesterol from the bloodstream, and NOTCH2, which is involved in cell-to-cell communication and is important during development.
Dysfunction in surface protein recycling is implicated in several conditions, including Alzheimer’s disease and high cholesterol.

To analyze retriever’s structure, the researchers expressed human proteins in armyworm cells, a common method for generating protein samples. They then revealed its 3D structure using cryo-electron microscopy and employed AlphaFold Multimer, an open-source program developed by Google and academic partners that applies artificial intelligence to the amino acid sequences of proteins to predict how they fold and assemble into protein complexes.
They found that retriever resembles another recycling complex known as retromer, but with some striking physical differences. Compared with retromer, retriever is more compact and twisted, has less negative charge on its surface, and has a short tail, dubbed a “belt,” that wraps around retriever and blocks some of its surface.
“The belt sequence is one striking difference between retriever and retromer,” Dr. Burstein said.
Retriever binds to a group of 12 other proteins, collectively called CCC, which form a striking pentagram star or ring structure. “Once you can see this in 3D, you can see how one protein fits into another, almost like a mechanical engineer,” Dr. Burstein said.
Oddly, he said, the CCC component proteins have conserved differences from each other, raising the question of why the cell needs differences that have been maintained through evolution, rather than joining 10 identical molecules: “That remains a mystery,” Dr. Burstein said.
To test whether this system was consistent through evolution, the researchers investigated retriever-CCC shapes in fish, amoebas, and other species. They found “highly consistent” ring arrangements, indicating that this protein recycling system is long-established.
Two other research groups independently studied the retriever structure, proposing highly similar structural models, but they did not resolve the structure of retriever at the same high resolution that the UTSW team did, Dr. Burstein said.
“This work opens avenues for investigating many aspects of retriever-CCC in biology and disease,” the researchers concluded in the study.
Dr. Burstein holds the Berta M. and Cecil O. Patterson Chair in Gastroenterology.

