Cryo-EM reveals function of protein implicated in rare hereditary disease

Understanding the mechanisms of cystinosis – a rare and devastating hereditary disease that can appear in infancy – could lead to better treatments for that condition as well as a deeper understanding of cellular transport.
Researchers at UT Southwestern and in California used cryo-electron microscopy (cryo-EM) along with other cutting-edge biophysical techniques to reveal the structure and function of cystinosin, a key transporter for cystine, the two-molecule complex of the amino acid cysteine. They were able to define structures of the transporter protein in multiple conformations, including bound and unbound states, a first step toward understanding what goes wrong with the molecule in the genetic disease called cystinosis.
The disease that causes a buildup of cysteine crystals in the body affects fewer than 5,000 people in the United States, according to the National Institutes of Health (NIH). It is an autosomal recessive disorder, meaning it occurs when someone inherits one mutated cystinosin gene from each parent. Although the condition is present from birth, symptoms can appear at different ages and vary in severity.
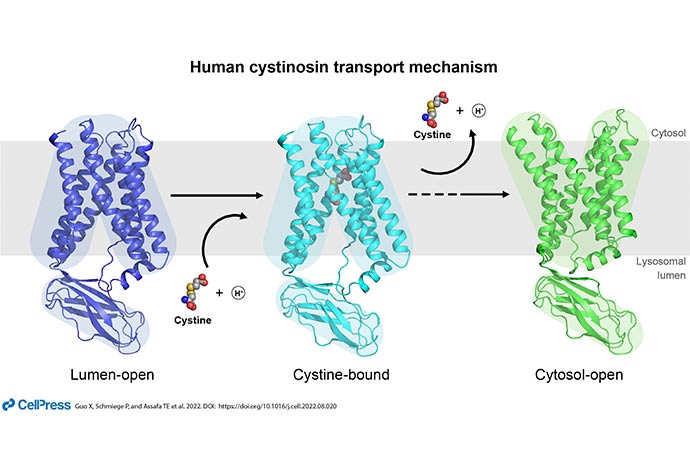
“Cystinosis, discovered in 1903, was long known as a genetic disease of lysosomal transport. But without a structure, scientists were unable to picture how the mutation led to the condition,” said Xiaochun Li, Ph.D., Associate Professor of Molecular Genetics and Biophysics and corresponding author of a study outlining this research in Cell.
Lysosomes are sac-like, membrane-bound organelles that hold enzymes the cell needs for digestion, housekeeping, and other functions. The amino acid cysteine is used to make proteins throughout the body. Most people adequately recycle this key amino acid.
Mutations that lead to an inability to recycle cysteine – in this case by rendering cystinosin unable to be transported out of the lysosomes – result in a buildup of crystals that are toxic to cells.
“This project incorporates several cutting-edge technologies from the field of biophysics. We collaborated with researchers at Stanford University and at UC Santa Cruz to present six structures at atomic resolution. The cryo-EM studies were all carried out at UT Southwestern’s cryo-EM core facility (CEMF),” Dr. Li said.
Among many intriguing observations, the researchers’ analyses determined the mechanism behind cystinosin’s shape change between its different states. In addition, while most mutations in the protein interrupted cystine transport, potentially leading to cystinosis, the researchers discovered a select few that improved the protein’s function. This could lead to novel therapeutics, they said.
Dr. Li said a side benefit of doing such work at an academic medical center is the opportunity to interact with learners.
“As a UT Southwestern faculty member, I am committed to training the next generation of scientists. In my lab, this project was led by Philip Schmiege, currently a fourth-year graduate candidate in the Molecular Biophysics program. He is an extremely talented individual with a natural ability to ask important scientific questions and carry out the research to the highest standard, which is evident by his work on this project,” Dr. Li said.
UTSW co-authors include: Rong Wang, Ph.D., a postdoctoral researcher; Linda Donnelly, manager in the lab of Joseph Goldstein, M.D., a Nobel Laureate and Chair of Molecular Genetics; Michael Fine, Ph.D., a former postdoctoral fellow; and Mr. Schmiege, all of Molecular Genetics. In addition to researchers at Stanford and UC Santa Cruz, scientists at the National Heart, Lung, and Blood Institute (NHLBI) of the NIH also participated.
The study received support from the NIH (R01 GM117108, R35GM131781, P01 HL160487, R01 GM135343, T32GM131963).
Dr. Goldstein, a Regental Professor, holds the Julie and Louis A. Beecherl, Jr. Distinguished Chair in Biomedical Research, and the Paul J. Thomas Chair in Medicine.
Dr. Li is a Rita C. and William P. Clements, Jr. Scholar in Biomedical Research at UT Southwestern.

