Breakthrough after breakthrough
Like the fictional Sherlock Holmes, UT Southwestern biochemist Dr. Zhijian “James” Chen approaches scientific mysteries by applying his keen observational skills and an uncanny ability to follow clues to their logical, satisfying conclusions. One key difference between them: Dr. Chen’s story continues to unfold.
Even as he was accepting the 2019 Breakthrough Prize in Life Sciences for solving one mystery in innate immunity, Dr. Chen was endeavoring to crack another case by carefully analyzing clues.
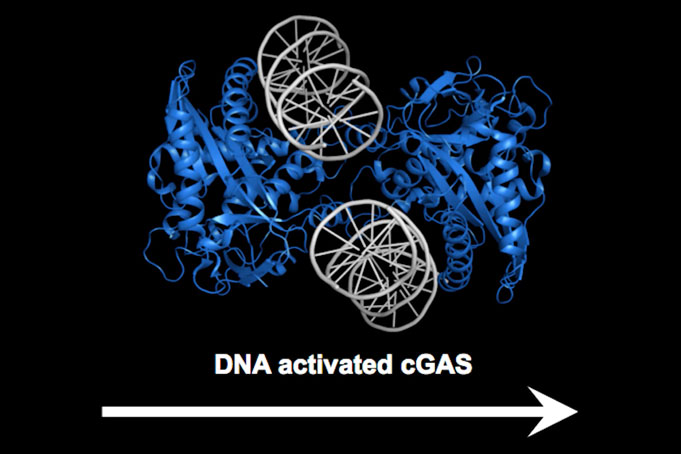
The $3 million Breakthrough Prize recognized Dr. Chen – Professor of Molecular Biology and Director of the University’s Center for Inflammation Research – for identifying the DNA-sensing enzyme cGAS (cyclic GMP-AMP synthase), which sounds the alarm to set off innate immunity inside cells in response to DNA that the enzyme encounters in areas of the cell where that genetic material should not exist. When the DNA is from an external source, it triggers immunity. When derived from an internal source, such as DNA that leaks out of the host’s nucleus or mitochondria, it can set off autoimmunity.
Dr. Chen identified cGAS and its secondary messenger cGAMP in his laboratory at UT Southwestern, helping strengthen the University as a world leader in research on innate immunity – the body’s first line of defense against disease.
“The impact of Dr. Chen’s breakthrough discovery of the cGAS pathway is continuing to increase. It is now apparent that this signaling system relays all sorts of damage signals associated with major diseases, including autoimmune diseases, cancer, and even Parkinson’s disease,” says Dr. Eric Olson, Chair of Molecular Biology and Director of the Hamon Center for Regenerative Science and Medicine.
New look at old question
Dr. Chen’s latest scientific advance, published in Nature in November, describes a different immune response pathway that resembles an ancient defense system used by plants.
“A long-standing question in this field is how one protein, NLRP3, can be activated by many diverse agents that don’t appear to share any chemical or structural similarities, including toxins and cholesterol crystals,” says Dr. Chen, a Howard Hughes Medical Institute Investigator who holds the George L. MacGregor Distinguished Chair in Biomedical Science and has a secondary appointment in the Center for the Genetics of Host Defense.
The NLRP3 protein is instrumental in the cell’s assembly of a multiprotein complex called the inflammasome, which in turn triggers the pathway for inflammatory cell death, or pyroptosis from the Greek “pyro” meaning fire. The inflammasome also increases the body’s production of immune modulators, such as interleukins, that aid in the body’s immune response.
In addition, the NLRP3 protein helps spark inflammation in a group of autoinflammatory diseases, including a form of brain-cell inflammation associated with Alzheimer’s disease.
Through a combination of biochemical, advanced imaging, and genetic approaches, the Chen lab revealed a previously unknown structural change within the cells. They showed that diverse stimuli all cause the cellular organelle called the trans-Golgi network to break apart into fluid-filled sacs, or vesicles. A special component contained in those vesicles binds to a specific region in NLRP3, triggering activation of the inflammasome.
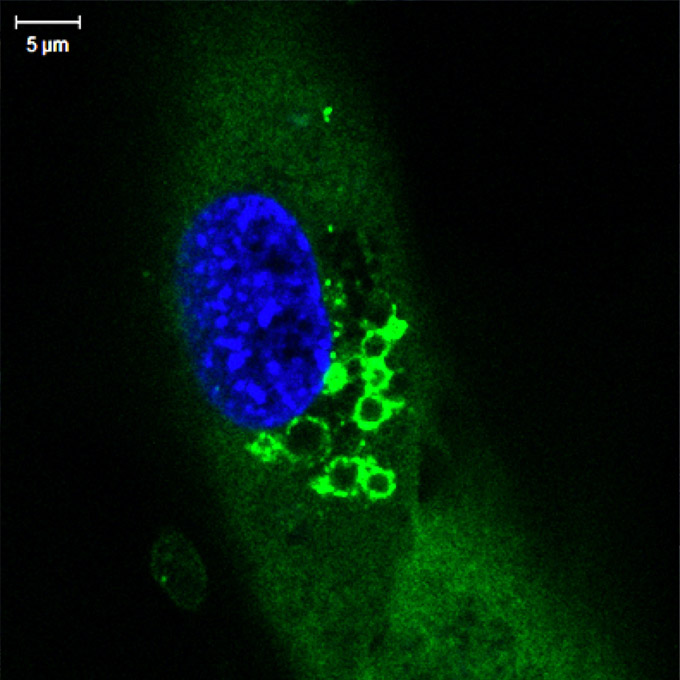
“The NLRP3 inflammasome is unique in that it can be triggered by a large array of stimuli,” Dr. Chen says. “In this study my postdoc Dr. Jueqi Chen found that rather than directly recognizing the noxious agents, the NLRP3 inflammasome detects a structural change in the host cell caused by a range of different agents that cause damage. In fact, NLRP3 activation is reminiscent of the ‘guard model’ that plants use to defend against diverse threats by monitoring host targets that have been altered, the so-called pathogen-induced-altered-self approach.”
Binding to the disassembled trans-Golgi network vesicles as the “altered self,” NLRP3 indirectly senses a large variety of pathogen- and danger-associated molecules, Dr. Chen concludes.
Those who know this sleuth of science describe the Nature story as classic James Chen: logically following the clues and revealing new insights.
Recruited to UTSW
“James was the first recruit to the Department of Molecular Biology after I launched the Department in 1996,” Dr. Olson recalls. “Having identified James as a promising young investigator, I needed help recruiting him, so Nobel Laureate Dr. Joe Goldstein stepped up for me, as he has done so many times. Together, he and I were able to lure James to Dallas, and the rest is history!”
Dr. Chen received the National Academy of Sciences (NAS) Award in Molecular Biology, a prestigious honor for promising early career investigators, and was elected to the NAS two years later. At a campus party celebrating that recognition, he joked that he had stayed busy from the moment he set foot on campus. After all, as the Department’s first faculty recruit, his laboratory was right next to that of his hardworking Chair, Dr. Olson.
True, his lab was and remains next door to that of Dr. Olson, holder of the Pogue Distinguished Chair in Research on Cardiac Birth Defects, The Robert A. Welch Distinguished Chair in Science, and the Annie and Willie Nelson Professorship in Stem Cell Research. But proximity as the reason for his hard work? Not so much.
Dr. Chen grew up in an isolated southern Chinese village.
“I’d never seen a TV, there were very few cars, but I was always curious about nature. I didn’t know what science was,” he says, laughing. His mother, an elementary school teacher, and his father both encouraged his interest in science, math, and physics, and he excelled at those subjects.
By the time he went off to college, he realized that becoming a scientist was a highly honorable profession and he says he feels fortunate to have pursued his career, which began with an undergraduate biology degree at Fujian Normal University. By placing first on a biochemistry exam, he received an overseas scholarship that took him to the State University of New York at Buffalo, where he earned a Ph.D.
Early in his career, he uncovered a new, unexpected role for the small protein ubiquitin, showing that it activates proteins important for inflammatory responses and other essential cellular functions.
League of his own
“We are systematically investigating these pathways in order to have a detailed understanding of how they work in microbial infections, in autoimmune diseases like lupus, and in cancer.”
In one of his earliest breakthroughs after arriving at UT Southwestern, Dr. Chen found that the cell’s energy-producing bodies – the mitochondria – contribute to the body’s immune response. In 2005, he identified the first mitochondrial protein known to be involved in immune defense against any microbial infections – MAVS (mitochondrial antiviral signaling) protein – and named it after the Dallas Mavericks basketball team. MAVS plays a pivotal role in the sensing pathway for RNA viruses such as hepatitis C, West Nile, SARS (severe acute respiratory syndrome), and influenza.
Since Dr. Chen arrived at UTSW in 1997, his work on innate immunity has encompassed studies that span RNA viruses, DNA viruses, autoimmune conditions such as lupus, Parkinson’s disease, Alzheimer’s disease, and cancer (read related story).
So what’s next?
“We are systematically investigating these pathways in order to have a detailed understanding of how they work in microbial infections, in autoimmune diseases like lupus, and in cancer,” he says. “That, basically, is the direction in which we and others are going.”
For autoimmune disease and inflammatory disease, that direction might lead to developing inhibitors that block the pathway. For cancer, the goal would be to do the opposite: to find ways to activate the pathways to enhance the immune system’s response to malignancies.
Looking back on Dr. Chen’s arrival in Texas, Dr. Olson says: “It was impossible to anticipate where James’ work would lead, and it took him some time to settle on his ultimate path.
“What was most important was that he was exploring a fundamental process of intracellular signaling in a unique and rigorous way – trying to simplify the system in a test tube. One thing led to another, and now we are seeing the unpredictable and powerful outcome of basic hypothesis-driven research.”
“My goal has always been to try to solve problems. Once we make a new discovery, we want to understand how things work and then move on to the next discovery,” Dr. Chen says.
Dr. Goldstein, a Regental Professor, is Chair of Molecular Genetics and a Professor of Molecular Genetics and Internal Medicine. He holds the Julie and Louis A. Beecherl, Jr. Distinguished Chair in Biomedical Research and the Paul J. Thomas Chair in Medicine.
Dr. Hobbs holds the Philip O’Bryan Montgomery, Jr., M.D. Distinguished Chair in Developmental Biology, Eugene McDermott Distinguished Chair for the Study of Human Growth and Development, and the 1995 Dallas Heart Ball Chair in Cardiology Research.

