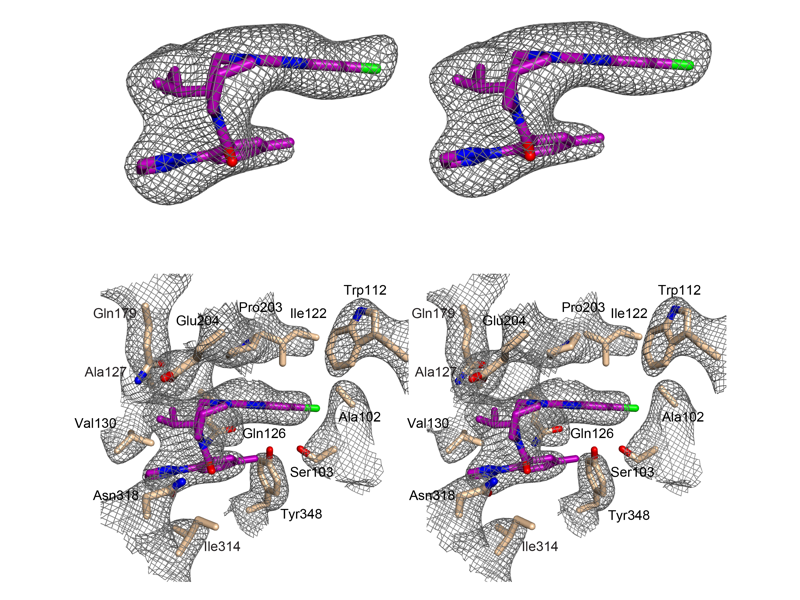
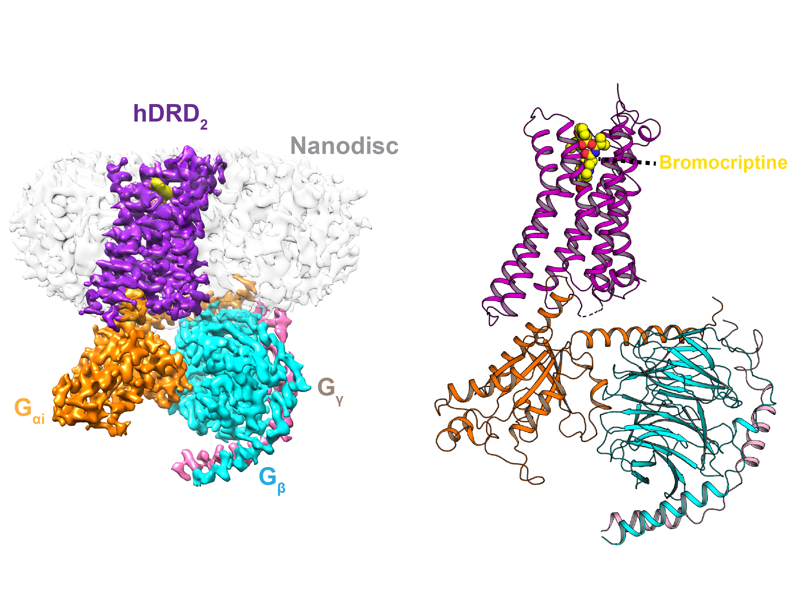
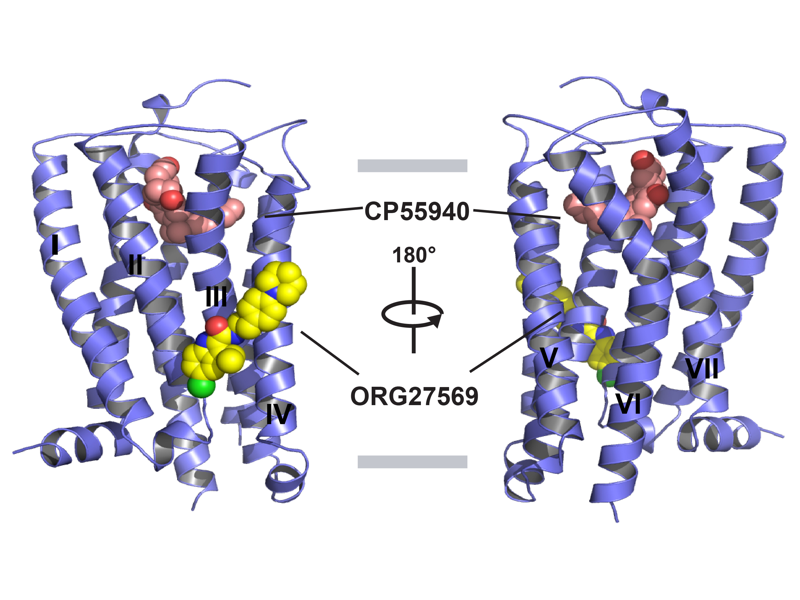
The Rosenbaum Lab:
Membrane Protein Signaling
The Rosenbaum Lab studies the structure and function of integral membrane signaling proteins involved in human disease.
We use a diverse set of biochemical and biophysical techniques to unravel the molecular mechanisms of GPCR signaling and lipid homeostasis, and also seek to translate our fundamental insights into new therapeutic strategies.

About the Lab
The lab is directed by Daniel Rosenbaum at UT Southwestern Medical Center in the Department of Biophysics.

Recent Publications

Contact
Get In Touch
Rosenbaum Lab
Department of Biophysics
University of Texas Southwestern Medical Center
North Campus ND10.504
6001 Forest Park Road
Dallas, Texas 75390